Important Risk Information
Edwards SAPIEN 3, Edwards SAPIEN 3 Ultra, and Edwards SAPIEN 3 Ultra RESILIA Transcatheter Heart Valve System
Indications:
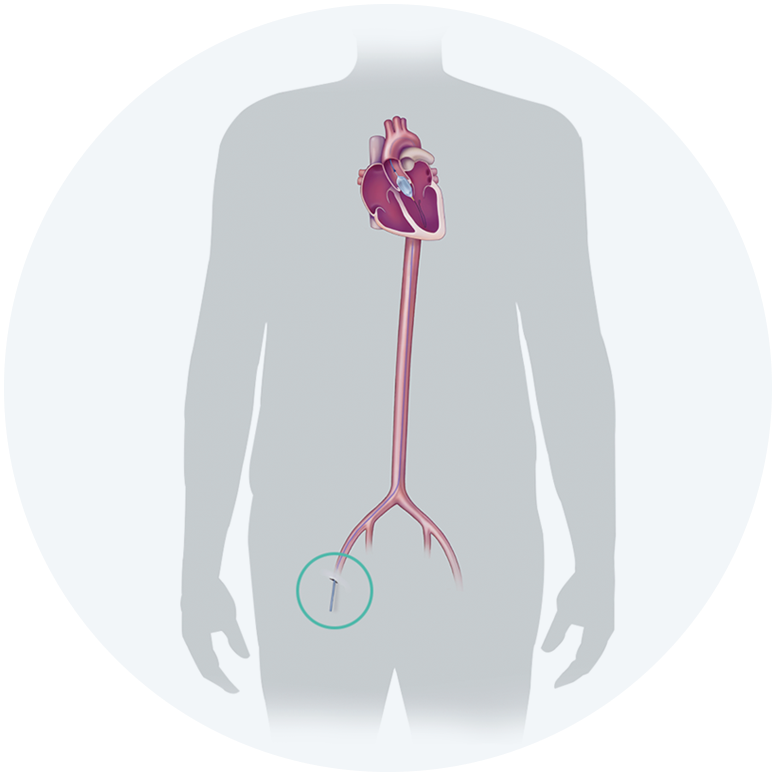
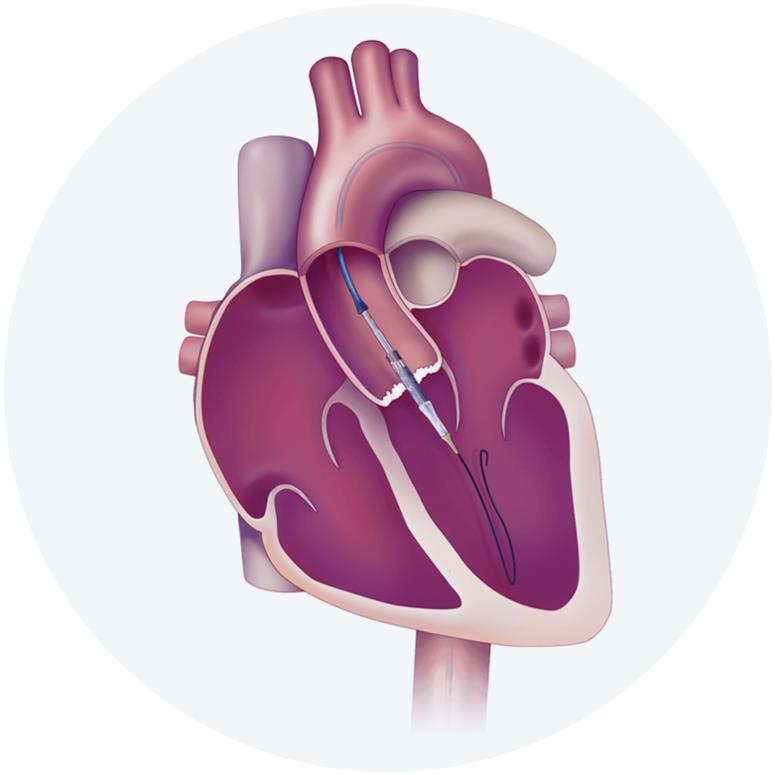
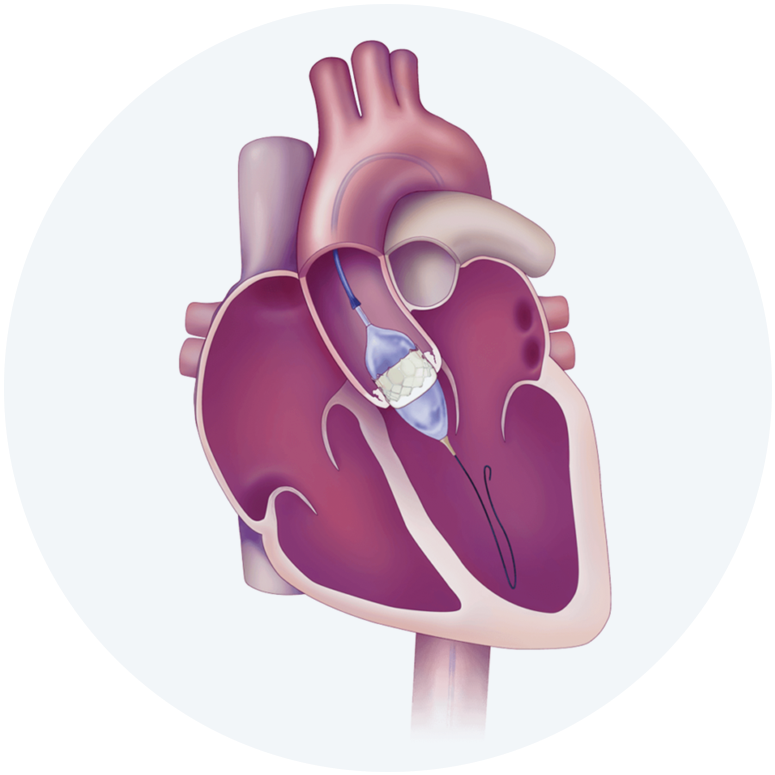
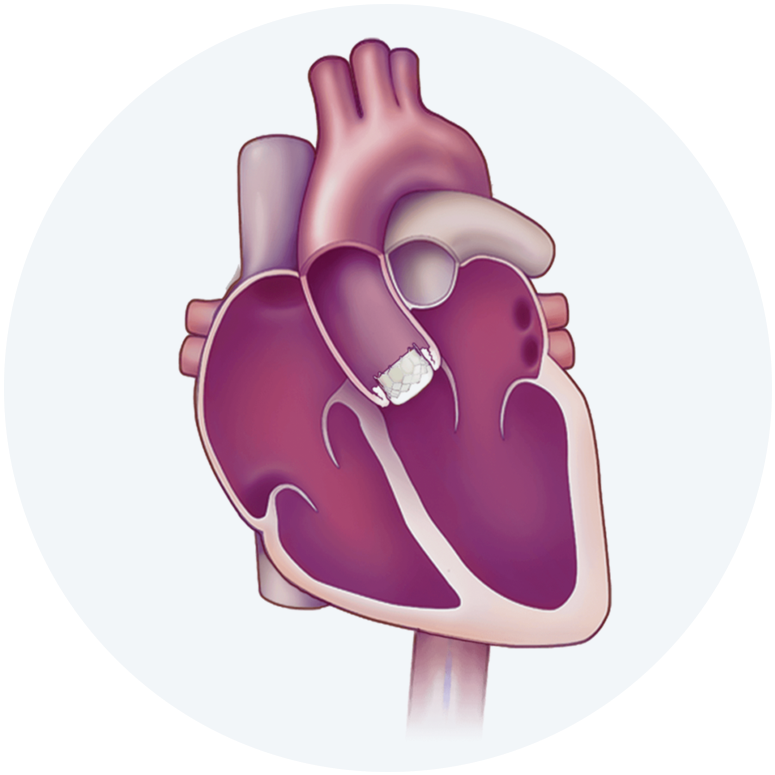
The Edwards SAPIEN 3, SAPIEN 3 Ultra and SAPIEN 3 Ultra RESILIA Transcatheter Heart Valve system is indicated for relief of aortic stenosis in patients with symptomatic heart disease due to severe native calcific aortic stenosis who are judged by a Heart Team, including a cardiac surgeon, to be appropriate for the transcatheter heart valve replacement therapy.
The Edwards SAPIEN 3, SAPIEN 3 Ultra and SAPIEN 3 Ultra RESILIA Transcatheter Heart Valve system is indicated for patients with symptomatic heart disease due to failing (stenosed, insufficient, or combined) of a surgical or transcatheter bioprosthetic aortic valve, a surgical bioprosthetic mitral valve, or a native mitral valve with an annuloplasty ring who are judged by a Heart Team, including a cardiac surgeon, to be at high or greater risk for open surgical therapy (i.e., predicted risk of surgical mortality ≥ 8% at 30 days, based on the Society of Thoracic Surgeons (STS) risk score and other clinical co-morbidities unmeasured by the STS risk calculator).
Contraindications (Who should not use):
The Edwards SAPIEN 3, Edwards SAPIEN 3 Ultra and SAPIEN 3 Ultra RESILIA Transcatheter Heart Valve System should not be used in patients who:
- Cannot tolerate medications that thin the blood or prevent blood clots from forming.
- Have an active infection in the heart or elsewhere.
- Have a mitral ring that is damaged and can no longer support the valve.
Warnings:
The Edwards SAPIEN 3, Edwards SAPIEN 3 Ultra and SAPIEN 3 Ultra RESILIA Transcatheter Heart Valve System should not be used in patients who:
- There may be an increased risk of stroke in transcatheter aortic valve replacement procedures, compared to other standard treatments for aortic stenosis in the high or greater risk population.
- If an incorrect valve size for your anatomy is used, it may lead to heart injury, valve leakage, movement, or dislodgement.
- Patients should talk to their doctor if they have significant heart disease, a mitral valve device or are sensitive to anesthesia, contrast media, cobalt, nickel, chromium, molybdenum, titanium, manganese, silicon, and/or plastics.
- The Edwards SAPIEN 3 Ultra, SAPIEN 3 Ultra RESILIA and SAPIEN 3 valves may not last as long in younger patients, or patients with a disease that results in more calcium in their blood.
- During the procedure, your doctors should monitor the dye used in the body; if used in excess it could lead to kidney damage. X-ray guidance used during the procedure may cause injury to the skin, which may be painful, damaging, and long-lasting.
- Patient’s creatinine level should be measured prior to the procedure.
- Patients who have already had a valve replaced should be carefully assessed by their physician prior to receiving a new valve to ensure proper placement of the new valve.
- Injury can occur if the delivery system is not used properly.
- Transcatheter heart valve patients should talk to their physicians about the potential need for medications that thin the blood or prevent blood clots from forming. Patients who do not may be at increased risk of a stroke. Blood-thinning medication may increase the risk of bleeding in the brain (stroke).
- Transcatheter valve replacement is not recommended in previous mitral valve rings that are damaged or have become too rigid.
Precautions:
The long-term durability of the Edwards SAPIEN 3 Ultra, SAPIEN 3 Ultra RESILIA and SAPIEN 3 transcatheter heart valves are not known at this time. Regular medical follow-up is recommended to evaluate how well a patient’s heart valve is performing. Limited clinical data are available for transcatheter aortic valve replacement in patients who are born with an aortic heart valve that has only two leaflets and who are determined to be at low risk for open heart surgery. A patient’s anatomical characteristics should be considered by their physicians when using the valve in this patient population. In addition, patient age should be considered as long-term durability of the valve has not been established. Patients who need a dental procedure should talk to their doctor about risk of infection and needing antibiotics. Patients should be treated post-procedure for heart infection as a precaution.
The safety and effectiveness of the transcatheter heart valves are also not known for patients who have:
- An aortic heart valve that is not calcified, contains only one leaflet, has leaflets with large pieces of calcium that may block the vessels that supply blood to the heart or in which the main problem is that the valve leaks.
- Who have a prosthetic ring in the tricuspid position.
- A heart that does not pump well, has thickening of the heart muscle, with or without blockage, unusual ultrasound images of the heart that could represent irregularities such as a blood clot, a diseased mitral valve that is calcified or leaking, or Gorlin syndrome, a condition that affects many areas of the body and increases the risk of developing various cancers and tumors.
- Low white, red or platelet blood cell counts, or history of bleeding because the blood does not clot properly.
- Diseased, abnormal, or irregularly shaped vessels leading to the heart. Vessels which are heavily diseased or too small for the delivery devices, or a large amount of calcification at the point of entry.
- Allergies to blood-thinning medications or dye injected during the procedure.
- Whose previously implanted artificial valve or ring is not securely in place or is damaged that could cause it to leak.
- Whose previously implanted valve or ring could block a blood vessel caused from the leaflet partially detaching.
Potential risks associated with the procedure include:
- Death, stroke, paralysis (loss of muscle function), permanent disability, or severe bleeding.
- Risks to the heart, including heart attack or heart failure, sudden loss of heart function, a heart that does not pump well, irregular heartbeat that may result in a need for a permanent pacemaker, chest pain, heart murmur, false aneurysm, recurring aortic stenosis (narrowing), too much fluid around the heart, injury to the structure of the heart.
- Risks to your lungs or breathing, including difficulty breathing, fainting, dizziness, buildup of fluid in or around the lungs, weakness, or inability to exercise.
- Risks involving bleeding or your blood supply, including formation of a blood clot, high or low blood pressure, limited blood supply, a decrease in red blood cells, or abnormal lab values, bleeding in the abdominal cavity, collection of blood under the skin, serious damage to the arteries, severe bleeding in the heart or in the body that could require a transfusion or surgery.
- Additional risks, including life-threatening infection, dislodgement of calcified material, air embolism (air bubbles in the blood vessels), poor kidney function or failure, nerve injury, fever, allergic reaction to anesthesia or dye, reoperation, pain, infection, or bleeding at incision sites, or swelling.
Additional potential risks specifically associated with the use of the heart valves include:
- Valve movement after deployment, blockage or disruption of blood flow through the heart, need for additional heart surgery or emergency heart surgery and possible removal of the Edwards SAPIEN 3 Ultra, SAPIEN 3 Ultra RESILIA and SAPIEN 3 valves, a blood clot that requires treatment, damage to the valve (e.g., wear, breakage, recurring aortic stenosis), valve issues not related to structure (e.g., leakage, inappropriate sizing or positioning, blockage, excess tissue in growth, blood cell damage) or mechanical failure of the delivery system and/or accessories.
CAUTION: Federal (United States) law restricts these devices to sale by or on the order of a physician.

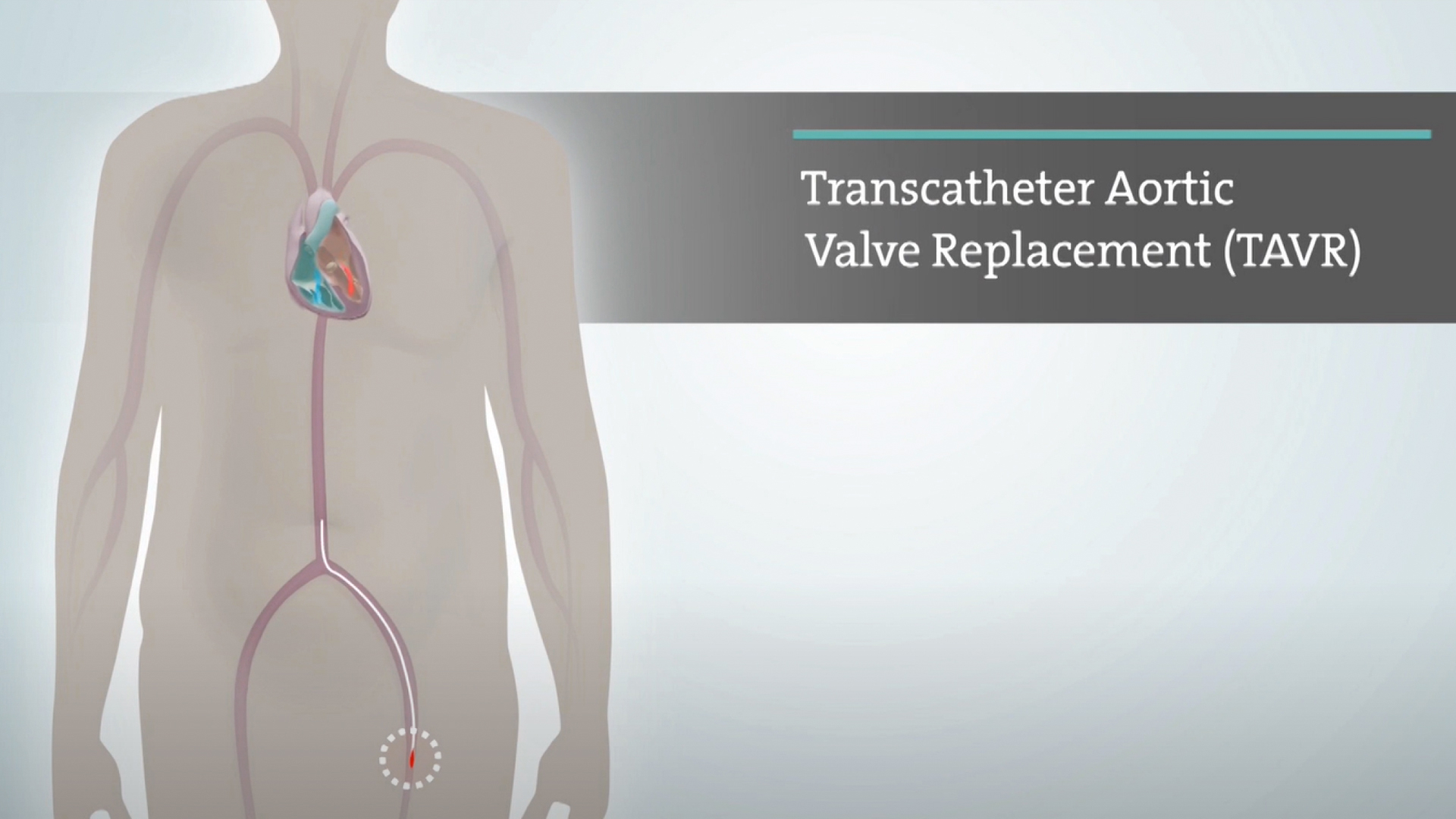




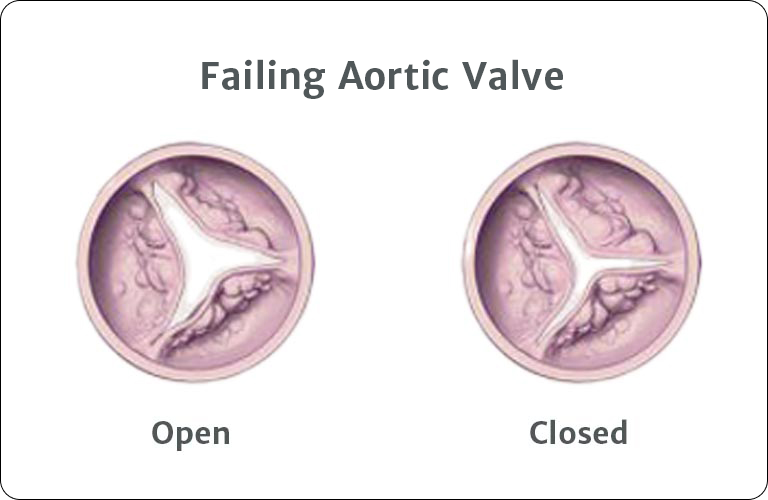
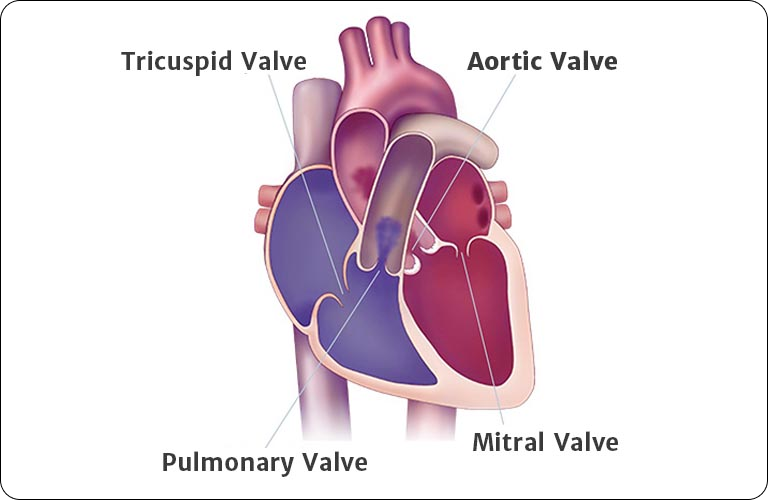
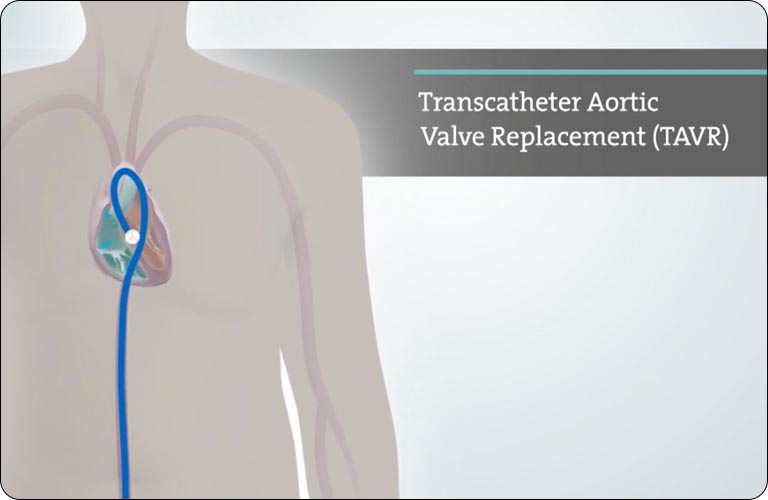

 Australia
Australia
 Brazil
Brazil
 Canada - French
Canada - French
 China - Taiwan
China - Taiwan
 Denmark
Denmark
 Finland
Finland
 Germany
Germany
 Italy
Italy
 Netherlands
Netherlands
 New Zealand
New Zealand
 Norway
Norway
 South Korea
South Korea
 Southeast Asia
Southeast Asia
 Sweden
Sweden
 United Kingdom
United Kingdom
 United States
United States