Without treatment, up to 50% of patients with severe aortic stenosis will die within an average of 2 years after symptoms appear.2
Learn moreDangers of Heart Valve Failure
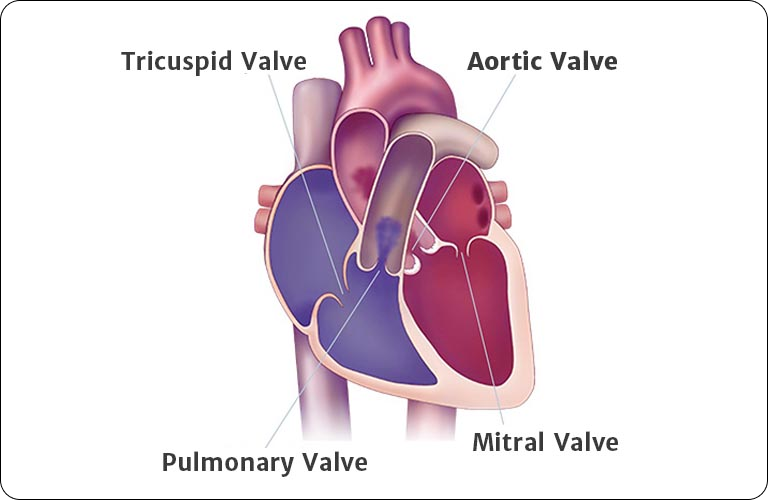
Aortic stenosis is the most common type of heart valve disease, affecting millions of people in the U.S. It is common in people 65 and older and affects 1 in 8 people over 75. This type of valve disease means your aortic valve cannot fully open or close like it should. Over time, the leaflets become stiff, which reduces their ability to fully open and close. When the leaflets don’t fully open, your heart must work harder to push blood through the aortic valve to your body. As a result, less oxygen-rich blood flows from the lungs to the brain and the rest of the body, which may cause symptoms. Severe aortic stenosis with symptoms, commonly known as heart valve failure, can be life-threatening if left untreated. That is why it is important to talk to your doctor at every appointment about the right time for treatment.
Does heart valve disease get worse over time?
Because aortic stenosis (heart valve disease) is progressive, it will get worse over time. Doctors typically measure it as mild, moderate, or severe. The stage depends on how damaged your aortic valve is. It is important to ask your doctor the stage of your disease at each appointment.
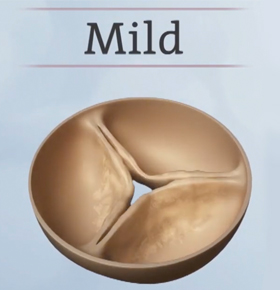

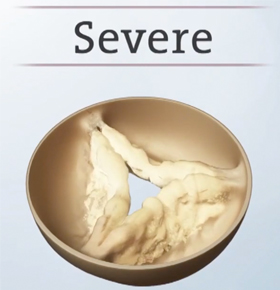
Mild and moderate stages
In the mild and moderate stages of heart valve disease, the decrease in blood flow is usually not significant enough to cause outward symptoms. In fact, many people are unaware they have the condition or may be told they have a heart murmur during a routine check-up.
Ask your doctor for an echocardiogram —a non-invasive test that can tell you how well your heart valves are working. The American College of Cardiology and American Heart Association recommend that people with mild aortic stenosis get an echocardiogram every 3-5 years and people with moderate aortic stenosis get an echocardiogram every 1-2 years.1

Severe stage
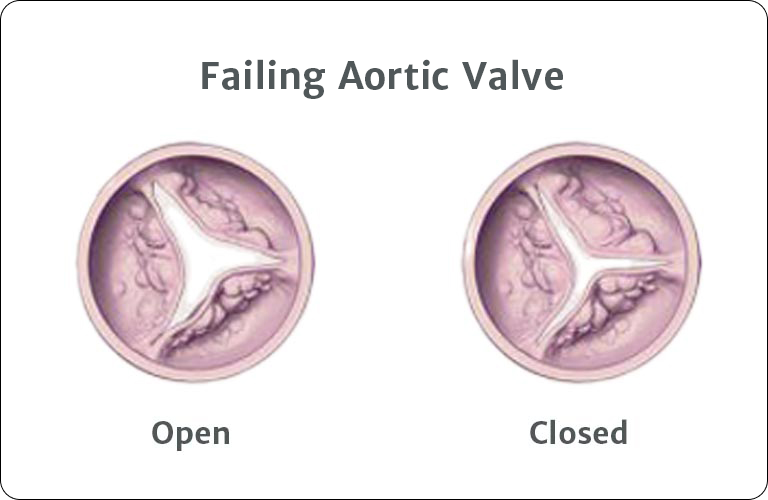
Once heart valve disease advances to the severe stage, it can be deadly. This stage is called severe aortic stenosis, commonly known as heart valve failure. As the leaflets become more damaged, the opening of the aortic valve becomes more narrowed, and your heart muscle gets weaker. Uncomfortable symptoms such as shortness of breath and chest pain may become more noticeable and can become life-threatening. Patients sometimes confuse these symptoms with normal signs of aging. It is important to seek treatment right away. Don’t wait.

Why is heart valve failure dangerous?
Heart valve failure is dangerous and delaying treatment can be deadly. At this advanced stage, the aortic valve has a severe buildup of calcium, and it has a difficult time opening and closing. When this happens, your risk for heart failure significantly increases.
You may think it is ok to delay talking to your doctor about new or worsening symptoms, but it is important to share this information once you first notice changes. Do not underestimate heart valve failure.
When is the right time for heart valve failure treatment?
It is understandable to want to wait for just the right time, but it is important to note that it doesn't exist. If you have heart valve failure and are experiencing symptoms, the time is now. Don't wait. Be an active participant in your heart health and ask your doctor about your treatment options at your next appointment.
The American College of Cardiology and the American Heart Association recommend that patients receive treatment as soon as they are diagnosed with severe aortic stenosis and start to experience symptoms.1

What are the symptoms of heart valve failure?
Mild to moderate aortic stenosis (heart valve disease) often causes no outward symptoms. However, as the disease progresses, you may begin to feel symptoms. Once symptoms appear heart valve failure can be deadly if untreated. That’s why it is important that you talk to your doctor about any new or worsening symptoms, including changes in your daily activities. These symptoms may not be due to aging, but rather heart valve failure.
Symptoms may include:
- Shortness of breath
- Chest pain
- Fatigue (low energy)
- Lightheadedness, feeling dizzy, and/or fainting
- Difficulty in walking short distances
- Swollen ankles and feet
- Rapid, fluttering heartbeat
These symptoms may mean your body is not getting enough oxygen. Over time, you may feel tired and weak. These may be signs that your heart valve failure has reached a life-threatening point.
Don’t confuse heart valve failure symptoms with signs of aging
If you're feeling fatigue, dizziness, or shortness of breath, it could be heart valve disease, not age. Many patients mistakenly think that heart valve failure symptoms are normal signs of aging. Studies have shown that while many aortic stenosis patients initially report no symptoms, after closer examination, 32% do have symptoms.3
If you've been diagnosed with aortic stenosis (heart valve disease) and experience any of the symptoms of severe aortic stenosis (heart valve failure), talk to your doctor about whether it is time for treatment. Don't wait.

Your future belongs to you, not to your heart valve failure
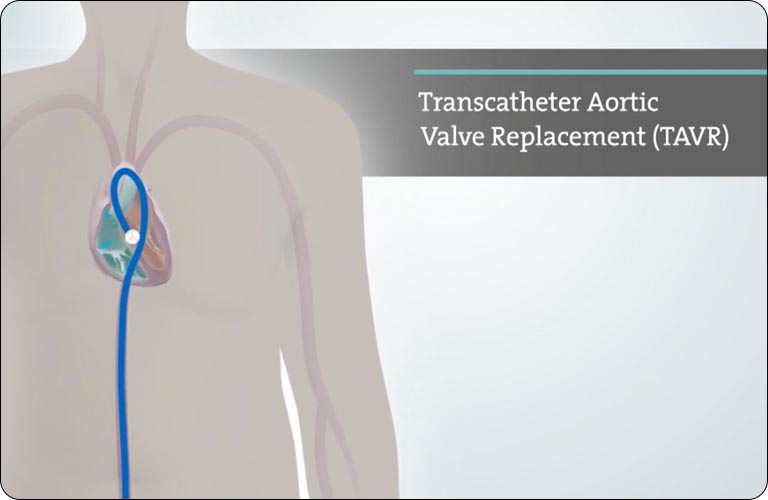
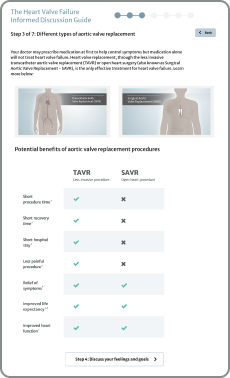
If you've been diagnosed with severe aortic stenosis (heart valve disease) and you have symptoms, then the time for treatment is now. Ask your doctor for a TAVR evaluation. Or find a TAVR Hospital near you, where you will be evaluated for all your treatment options.
Find a TAVR Hospital
Patient Stories
Former Police Chief Tate took control over his heart valve failure
Chief Tate described his heart valve failure symptoms, how they impacted his day-to-day, and why he talked to his doctor about treatment.
Watch Chief Tate’s story
Want more information delivered directly to you?
Get a free information kit by email or mail to learn about heart valve failure and the TAVR procedure.
What you’ll receive in your kit:
- Education on heart valve failure and symptoms checklist
- Information on TAVR as a treatment option
- Videos of patients sharing their experiences with TAVR
- Discussion guide for talking with your doctor
- List of hospitals in your area that perform TAVR
References
1. Otto CM, Nishimura RA, Bonow RO, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;143:e72-e227.
2. Otto, C VALVE DISEASE; Timing of aortic valve surgery. Heart. 2000;84(2):211-218.
3. Das P, Rimington H, Chambers J. Exercise testing to stratify risk in aortic stenosis. Eur Heart J. 2005;26(13):1309-1313.


 Australia
Australia
 Brazil
Brazil
 Canada - French
Canada - French
 China - Taiwan
China - Taiwan
 Denmark
Denmark
 Finland
Finland
 Germany
Germany
 Italy
Italy
 Netherlands
Netherlands
 New Zealand
New Zealand
 Norway
Norway
 South Korea
South Korea
 Southeast Asia
Southeast Asia
 Sweden
Sweden
 United Kingdom
United Kingdom
 United States
United States